There is considerable interest in the development of reduced protein diets balanced with supplemental crystalline amino acids for broiler chickens due to economic, environmental and bird welfare advantages (Moss et al., 2018).
However, reduced protein diets may result in dietary amino acids being redistributed away from growth and production processes, toward intestinal cells involved in immune and inflammatory responses (Le Floc’h etal., 2004). In addition, an unbalanced supply of amino acids (AA) in the diet can be deleterious to the immune system (Li et al., 2007).
Thus, an ideal balance of AA is crucial for broiler chicken production in particular if birds are reared without antibiotics. All of the crystalline AA supplemented in commercial poultry production are in their natural form (L-form) except methionine (Met) (Esteve-Garcia and Khan, 2018). In poultry diets, Met is the first limiting amino acid and the dietary supplemental Met sources include L-Methionine (L-Met; 99% purity), its synthetic forms DL-methionine (DL-Met, 99% purity) and liquid DL-2-hytroxy-4-methylthio butanoic acid (DL-HMTBA, containing 88% of active substance).
All three sources of methionine are currently supplemented in poultry diets to meet birds total sulfur amino acids (TSAA) requirements.
Met Metabolism and Function
Met is an essential AA involved in multiple fundamental biological processes, including protein synthesis, transmethylation and the synthesis of homocysteine. Apart from protein synthesis, Met is the major donor of the methyl group to affect DNA and protein methylation in cells including creatine production (Wu, 2013).
High dietary arginine has been recently demonstrated to improve chicken gut health (Bao, 2019) and creatine concentration in chicken breast meat (Chamruspollert et al, 2002) but possible depressed chicken performance might be due to increased dietary Met requirement (Chamruspollert et al., 2002).
Homocysteine is a key substrates in three additional essential reactions: (1) the recycling of intracellular folic acids;(2) the catabolism of choline and betaine; and (3) the transsulfuration pathways to produce cysteine (Cys) (Finkelstein, 1998).Consequently, the minimal daily requirement for Met varies as a function of the availability of cysteine, choline or betaine, Vitamin B12 and folic acid but cannot be replaced by choline or betaine in producing immune responses.
Because a portion of dietary Met is normally converted to Cys, it suggests that dietary Cys can spare, reduce, or replace a portion of the requirement for Met by as much as 50%-80% in birds (Shoveller et al., 2005). However, The Met portion used for Cys biosynthesis is only 81% on a dietary concentration basis, indicating the magnitude of response to Met supplementation when Cys is also deficient is less than that when Met is singly deficient (Baker, 2009).
Cys is the precursor of glutathione (GSH) and hydrogen sulfide (H₂S) (a signalling molecule) in animal cells, positively correlated with glutathione concentrations in the liver, spleen and muscle, playing an important role in regulating cellular signalling pathways in response to immunological challenges (Li et al., 2007). Cys preferably participates in the synthesis of keratin in feathers comparing to nutrient deposition in the breast muscle (Bonato, 2011). Glutathione is essential for normal intestinal function and the deficiency of glutathione will increase the susceptibility to carcinogenesis, oxidative injury.
It is noteworthy that taurine is an end product of TSAA with various physiological roles including conjugation with bile acids, stabilization of the cellular plasma membrane and a major antioxidant to regulate the cellular redox state (Hagiware et al., 2014). However, in some circumstances, taurine cannot be sufficiently synthesized in the liver although plasma Met and Cys concentrations are high. Recently it was found that supplementation of branched chain amino acids (BCAA) may improve taurine biosynthesis in the liver. Thus, the higher dietary BCAA levels may help TSAA to fully play their functional roles.
Commercially available L-Met is produced by bacteria fermentation and can be directly used to synthesize protein, provide methyl group or degrade through pathways to produce Cys. For DL-Met, it contains 50% D-Met and 50% LMet. In the chicken body, its D-Met is oxidatively deaminated to the α-keto analogue of L-Met, 2-keto-4 methylthio butanoic acid (KMB) by D-amino acid oxidase. It is assumed to be 100% efficacy, but it has been traditionally accepted that DL-Met is 95% efficacy relative to L_met due to equal dietary contributions of the D-and L-isomers (Baker, 2006).
Then KMB is converted to L-Met by transaminase (Brachet and Puigserver, 1992). For DL-HMTBA, it contains 50% L-HMTBA and 50% D-HMTBA. Its D-HMTBA is oxidized to KMB by L-2-hydroxy acid oxidase and D-HMTBA is dehydronised to KMB by D-2-hydroxy acid dehydrogenase. Then KMB is converted to L-Met by transaminase (Dibner and Knight, 1984). The key enzyme, D-amino acid oxidase exists only in the liver or the kidney and D-Met is not utilized directly by the cells of the gastrointestinal tract (Shen et al., 2015).
Relative Bioavailability Values of Met From Different Sources.
The evaluation of relative bioavailability values (RBV) of Met sources in broiler chickens has remained controversy for more than 50 years differences in experimental designs, methionine requirements, supplemental methionine levels, dietary factors such as dietary lysine, arginine, cysteine and branched chain amino acids concentrations, dietary energy levels, response criteria, the age of broiler chickens and statistical models. Based on a dose-response trial, the slope-ratio and non-linear multiple regression models have been widely used in those RBV evaluation studies (Little et al., 1997).
For three sources of Met, in the slope-ratio multiple regression model, the following multilinear regression was applied:
Y = a + (b1X1 + b2X2 + b3X3)
In which y = growth performance ( body weight gain and FCR, a = intercept (growth performance achieved with the negative control), b1 =the slope of DL-Met line, b2 =the slope of L-Met line and b3 =the slope of liquid HMTBA line, X1 = intake of supplemental DL-Met (g/day/bird), X2 = intake of supplemental L-Met (g/day/bird) and X3 = intake of supplemental liquid HMTBA (g/day/bird). RBV of L-Met and liquid HMTBA to DL-Met were given by the ratio of slope coefficients, b2 : b1 and b3 : b1 , respectively.
For three sources of Met, in the non-linear multiple regression model with common plateau, the following non-linear regression was applied:
Y = a + b (1 ̶ e(c1X1 + c2X2 + c3X3))
In which y = growth performance ( body weight gain and FCR), a = intercept (growth performance achieved with the negative control), a + b = asymtote, c1 =the steepness coefficient for DL-Met , c2 =the steepness coefficient for L-Met and c3 =the steepness coeficient of liquid MHA line, X1 = intake of supplemental DL-Met (g/day/bird), X2 = intake of supplemental L-Met (g/day/bird) and X3 = intake of supplemental liquid HMTBA (g/day/bird). RBV of L-Met and liquid HMTBA to DL-Met were given by the ratio of steepness coefficients, c2 : c1 and c3 : c1 , respectively.
Based on the slope-ratio analysis, in broiler chickens (21 to 42 d ) exposed heat stress, the RBV of liquid HMTBA ranged from 67% (FCR) to 83% (weight gain) relative to DL-Met (Rostagno and Barbosa, 1995). Following the non-linear regression model, Lemme et al., (2002) reported 72% weight gain and 51% FCR (1 to 42 days) of liquid DL-HMTBA RBV compared to DL-Met.
However, based on predominantly not accessible or published data in non-peer-reviewed journals, Vázquez-Añón et al., (2006) argued that DL-Met and DL-HMTBA did not fit the same dose response profile, concluding that at lower or deficient dietary TSAA levels, DL-HMTBIA responses were lower than those of DL-Met, whereas at the commercial or above requiremental levels, DL-HMTBA outperformed those for DL-Met.
In these studies, the highest supplemental level of Met was 0.4%. On the basis of 1.13% and 1.02% digestible lysine in the starter (d1-d10) and grower (d 11-d 28) periods, respectively, an experiment was conducted to consist of a basal diet without Met addition, and 4 increasing Met doses for DL-HMBTA and DLMet resulting in TSAA/Lysine ratios from 0.62 to 0.73 in the starter phase and 0.59 to 0.82 in the grower phase. For the starter period, growth performance were not improved from 0.66 to 0.73 TSAA/Lysine ratio. For the grower period, performance parameters responded quadratically to either DL-Met or DL-HMTBA supplementation, showing better efficacy for HMTBA than DL-Met at higher TSAA levels (Agostini et al., 2016).
In this study, although separate plateau models were used as suggested by Kratzer and Little (2006), Hoehler (2006) insisted that it was correct to use either slope-ratio (linear response) or nonlinear models with common plateau depending on the data structure of the respective dose-response trial.
Based on a control diet containing digestible TSAA 0.56%, adding 0.095%, 0.190% and 0.285% of either L-Met or DL-Met resulted in TSAA/Lysine ratios from 0.44 to 0.67 (Shen et al., 2015). In this study, experimental data (1 to 21 d) were analysed by the slope-ratio multiple regression model. The RBV of L-Met ranged from 138% (weight gain) to 141% (FCR) relative to DL-Met. Surprisingly, adding 0.190% L-Met to the control diet (TSAA/Lysine ratio is 0.59) had reached the growth plateau and it was much lower than 0.78 suggested by Dozier and Mercier (2013). In this control diet, digestible Threonine/Lysine ,Isoleucine/Lysine and digestible Valine/Lysine is 0.63, 0.63 and 0.71, respectively. They were also much lower than Ross nutrient recommendation. Considering that maximal chicken body weight gain at 21 days of age was only 762 grams , other dietary factors might limit Met supplementation responses.
In a 37 days broiler chicken trial, on the basis of control diets containing digestible TSAA 0.54%, 0.52% and 0.50% in starter, grower and finisher periods, respectively, adding 0.05%, 0.10%, 0.15% and 0.20% of either L-Met or DL-Met resulted in TSAA/Lysine ratios from 0.44 to 0.61in starter period, 0.50 to 0.69 in grower period and 0.51 to 0.71 in finisher period, respectively (Esteve-Garcia and Khan, 2018). In this study, when experimental data in 37 days were analyzed by non-linear models with common plateau, the RBV of L-Met ranged from 112% (weight gain) to 130% (FCR) relative to DL-Met.
Interestingly, for broiler chickens fed purified diets, L-Met was more efficiently utilized than DL-Met and DL-Met in turn was superior to equimolar amounts of DL-HMTBA. However, in semi-purified diets continuing high proportion of natural L-Met provided by soy bean meal, this trend lost sensitivity (Smith, 1965). It is noticed that in this semi-purified, soybean meal provided 75% of dietary total methionine concentration, leaving a very small proportion of the methionine requirements to be provided by supplemental methionine sources.
Therefore, the higher dietary supplemental methionine levels or the control diet containing much lower natural L-Methionine are crucial for methionine RBV evaluation. Recently a dose-response trial was conducted in an Australian University to compare RBV of L-Met and DL-HMTBA relative to DL-Met. On the basis of the control diet containing 0.637% digestible TSAA, adding equimolar 0.138%, 0.276% and 0.414% either DL-Met, L-Met or DL-HMTBA led to digestible TSAA/Lysine ratio from 0.50 to 0.80.
Surprisingly, even adding equimolar 0.414% either DL-Met or DL-HMTBA did not reached the response plateaus. However, for L-Met, when TSAA:Lys ratio equalled to 74.9% and 74.2%, the optimal body weight gain and FCR were reached, respectively, confirming that L-Met has the highest bioavailability. It is noticed that in this trial, the highest supplemental Methionine concentration achieved 56% of the dietary total methionine requirement, resulting in the highest ileal digestibility of Met and other amino acids (Figure 1), indicating that supplementation of l-Methionine significantly reduced N excretion.
It is noteworthy that TSAA: Lys ratio of 74 to 75 is the current practice in broiler chicken production. The fact that supplementation of either DL-Met or DL-MHA did not reach the body weight gain response plateau, strongly suggests in practice, L-Met supplementation may at least improve FCR by two points.
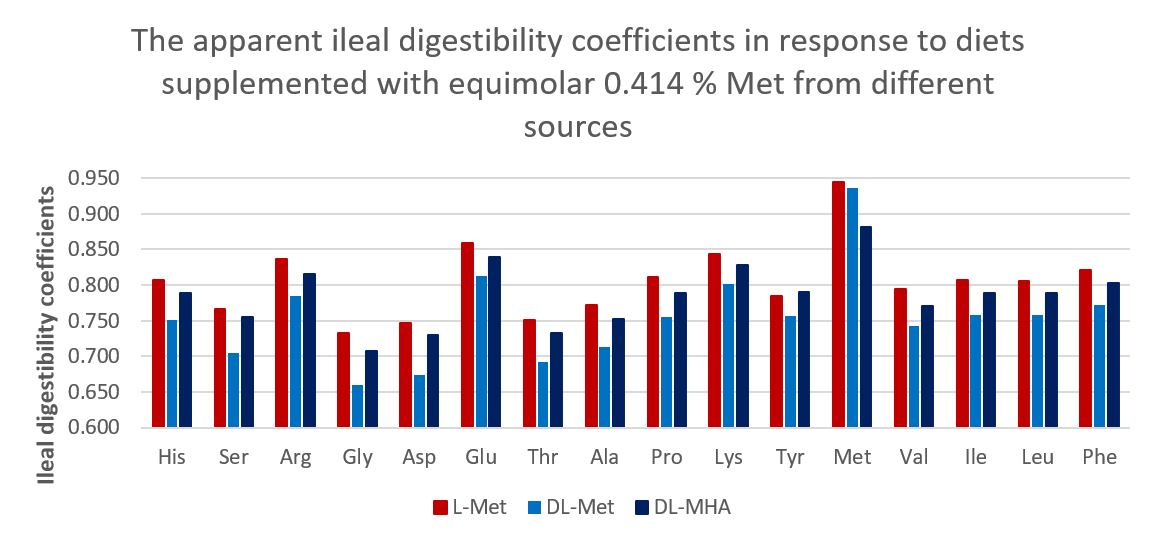
Figure 1. The apparent ileal digestibility coefficients in response to diets supplemented with equimolar 0.414% Met from different sources.

