Today is World Water Day, focusing on expediting efforts to address the global water and sanitation crisis. Since water is a fundamental need for everyone, it is essential that each one of us takes responsibility and takes action towards this cause.
In 2021 Redox commenced support for Médecins Sans Frontières (MSF) to aid their critical global efforts. Our donations help enable the teams at MSF to respond promptly to emergencies and deliver superior medical care to those in dire need-no more important than clean, accessible water.
MSF operates in countries where access to water can mean the difference between life and death. Inadequate water results in crop failure and suffering, while excess water leads to natural calamities like floods, landslides, and epidemics. Clean water is crucial in preventing waterborne illnesses.

Sera James, a resident of Akello village, shares a laugh with her friends as she draws water from a pump MSF teams have just installed to improve the community’s access to clean water during the dry season. Pibor country, South Sudan, March 2022.
NJIIRI KARAGO/MSF
During times of crisis and disaster, the most vulnerable, such as children, pregnant women, the elderly, and displaced individuals, are usually the first to bear the brunt. In Afghanistan and Syria, people are cut off from water supplies. In contrast, the Sahel region’s lack of food and water exacerbates existing inequalities. Eastern Africa experiences relentless storms and cyclones, causing widespread destruction.
Humanitarian medical needs continue to grow in these regions, and climate crises are emerging as a new area of concern for aid organizations. Drought in Madagascar is leading to increased malnutrition, while frequent extreme weather events are destroying livelihoods in countries such as Malawi. Persistent flooding in South Sudan has resulted in a rise in malaria cases.
These crises are often intertwined, with water being the common denominator.

MSF teams try out a water pump as part of a bid to provide clean water and latrines to displaced people in Rho camp—Democratic Republic of Congo, December 2021.
ALEXIS HUGUET
Despite the challenges facing the world’s freshwater resources, there is still hope for a sustainable future. By taking action to conserve and manage our water resources, we can help ensure a future where clean and safe water is available to everyone. We can make a difference by adopting sustainable water practices in our daily lives, such as reducing water usage, fixing leaks, and using water-efficient appliances. Organizations and governments can also play a significant role by implementing policies and initiatives that promote sustainable water management. Let us take inspiration from World Water Day to work together towards a future where access to fresh water is a reality for all.
Join us in supporting the critical work of Médecins Sans Frontières by clicking here to donate. Your contribution will help MSF continue providing lifesaving care in emergencies and beyond, ensuring that vulnerable communities receive the help they need even after the headlines have faded.
Produced from carbon-rich organic materials, activated carbon’s superior performance as an adsorbent lends itself to a growing list of applications as environmental regulations continue to become more stringent.
Activated carbon is a game-changing adsorbent, revolutionising how industries tackle contaminants and unwanted particles in liquids or gases. Pollution control efforts are particularly taking advantage of its capabilities, with reports pointing towards an optimistic future – all thanks to the growing call for environmental solutions globally.
How is Activated Carbon being used in industry?
Activated carbon is an incredibly versatile compound that serves a range of diverse functions.
- From the granular medium traditionally used to trap pollutants from liquids and gases to
- pellets designed explicitly for tackling volatile organic compounds (VOCs) and Hydrogen Sulfide (H2S) in gas filtration,
- it even comes in powder form – which can be added directly into liquid or soil to absorb toxins easily.
Its remarkable properties have enabled some incredible advancements across multiple industries.
While Activated carbon has become a powerful ally in multiple industries, from purifying potable and wastewater to cleaning out toxic gases from the air we breathe, it also plays an essential role in soil remediation by removing dangerous pollutants like PFAS and other organic compounds.
Even beverages can benefit– with Activated carbon able to strip wine and juices of their unwanted colour or toxins, such as patulin found commonly in apple juice!
Natures renewable resource
Customers are increasingly turning to sustainable solutions – unlike other forms of activated carbon, coconut shells and wood are renewable resources, each with unique properties when it comes to removing impurities.
In particular coconut shells are an incredibly sustainable solution to global raw material needs. Renewable and accessible within six months, coconuts outshine common alternative materials such as coal or wood that can take millions of years and decades to form. Not only do coconut trees capture CO2 from the atmosphere, but their harvesting is a by-product of our food industry – making them earth-friendly and beneficial in other ways.
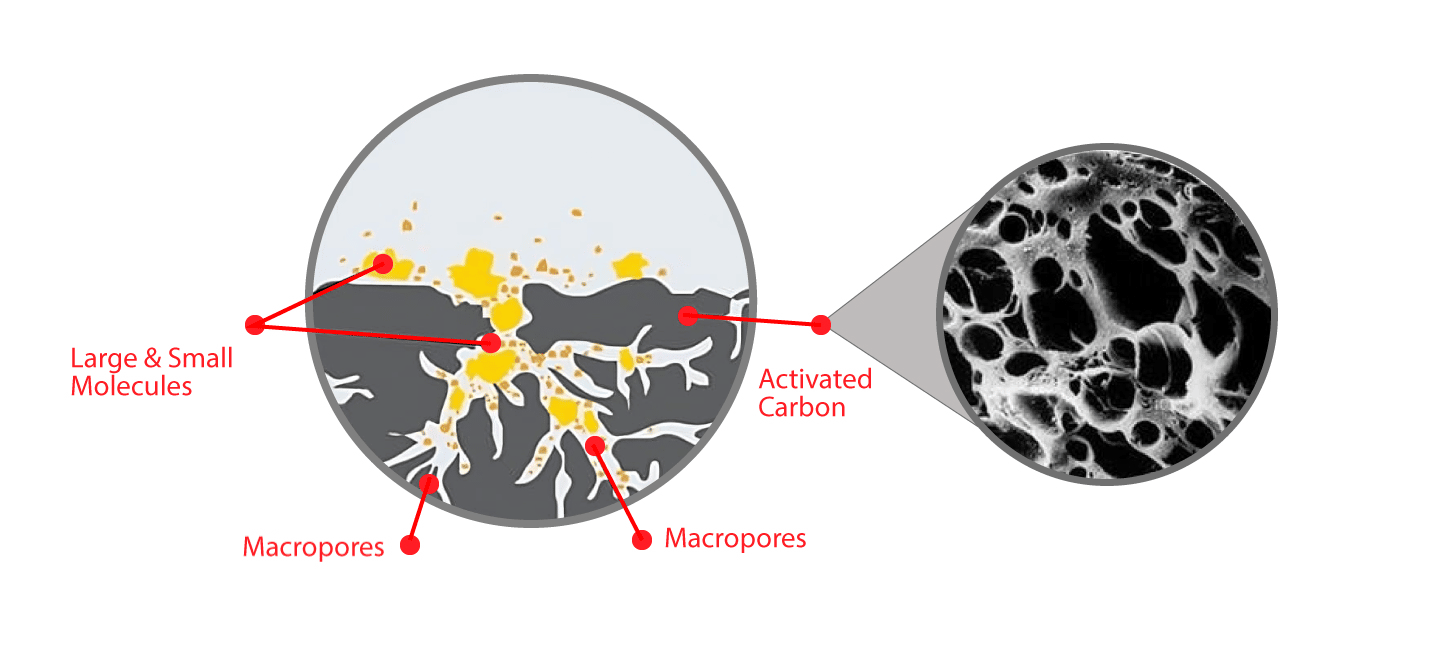
Activated carbon is an effective adsorbent because it is a highly porous material and provides a large surface area to which contaminants may adsorb.
Using it to produce activated carbon prevents it from being wasted and potentially burnt as fuel. In fact, every part of the coconut has a use, and there is no waste. The water, meat, husk and shell are all used across various industries.
Recent market reports point to an optimistic future driven by demand for its environmental uses. With a heightened awareness surrounding eco-friendly products like coconut-based activated carbon, new possibilities present daily as we uncover just how powerful this substance truly is in the fight against pollution.
How can we help?
Despite a range of competitor adsorbents available, activated carbon continues to lead the way across numerous applications. Thanks to solid growth prospects in key regions and industries, demand for this versatile material is set to remain healthy in the coming years. As a result, companies providing high-quality activated carbon will be well-positioned to capitalise on this trend.
Redox proudly offers a vast selection of activated carbon tailored to suit any application. From 25kg bags up to 500 kg bulker and with impregnation options – Redox only sources the highest quality carbons to suit your application through our trusted partner.
Unlock the potential of your sourcing strategy with Redox. Our experts are ready to help you realise a new level of success. Contact one of our experts today.
The importance of water is often overlooked in our modern world. National Water Week, hosted by the Australian Water Association (AWA), hopes to encourage individuals and communities to be inspired by how much we need this essential resource for life!
Difficulties in managing this precious resource are as varied as they are complex. Australia’s climate and landscape, coupled with the demands of agriculture and growing urban populations, can make water supply difficult. Northern Australia, for example, has significant water resources that are not easy to capture and store.
Add to this,
- Professional skills shortages
- Bushfire contamination
- Sewage and wastewater
The Australian Water Association is the most extensive not-for-profit network dealing in all aspects of water, including inspiring and driving a sustainable future for our country’s precious resource – fresh, clean drinking H2O! Through local, national and international networks of individual and business members, AWA shares information and opportunities to inspire positive change by sharing expertise.
How can Redox help?
Redox has a proud tradition of supplying quality water treatment products to all areas of the industry, reaching back more than fifty years. We know that innovation, safety, efficiency and dependability are paramount in treating one of the world’s most precious resources.
Our wide range encompasses items for use in all aspects of water treatment, from pH adjusters for potable drinking water, speciality biocides for cooling towers, Ion Exchange for power stations, and great-value pool chemicals. For the safest and cleanest water, we supply:
- Flocculants
- pH adjusters
- Fluoridation additives
- Organophosphates and biocides
- Filter media
- Scale inhibitors
- Coagulants
- Ion Exchange Resins
Browse our full water capability statement below or click here to download a copy.
Redox has been a member of the AWA for many years and has specialists in water treatment and filtration who can assist when you aren’t sure what to do. Whether it be floods, droughts or desalination, Redox is here to support our customers’ needs. Contact one of our specialists today to find out more.
Did you know that Hydrochloric Acid is also known as HCL, muriatic acid, or spirits of salt? It’s utilised in a variety of industrial and commercial settings. For those who work in industries that use this chemical, it’s vital to understand the most common applications, what they accomplish, and what you need to know to handle them safely and responsibly.
What Is Hydrochloric Acid?
Hydrochloric Acid is an odourless, colourless solution of hydrogen chloride in water with a pungent smell. But behind this almost invisible veneer lies a powerful punch. For instance, Hydrochloric Acid can react with metals to form an explosive gas. Yet, it can also be found in many home cleaning products.

1150 kg IBCs housed in our warehouse, classified Hazard Class 8, are for corrosive materials, defined as substances that can cause significant harm to living tissue and/or corrode steel and aluminium if they leak.
Hydrochloric Acid is classified as a class 8 hazardous product, i.e. it’s a corrosive substance and can cause burns and irritation to the skin. Due to its corrosive properties, extreme care must be taken when handling this product. Make sure to wear appropriate safety equipment when handling hydrochloric acid. Ensure to avoid direct eye contact; if this occurs, seek immediate medical advice.
We recommend you consult the safety data sheet when using, storing or handling the product.
Hydrochloric Acid in the market and its many uses
This potent acid is found in many industries and has a wide range of uses.
What is Hydrochloric Acid used for?
The most significant end uses for Hydrochloric Acid are cleaning, the productions of fertilisers and dyes, steel pickling, oil well acidising, food manufacturing, producing calcium chloride, and ore processing.
How it’s used also varies significantly. For instance, in water treatment, it’s used to control pH levels, or in swimming pools, it can help remove any stubborn algae from the floors and walls of your pool. In acidising oil wells, it helps remove carbonate reservoirs, or limestones and dolomites, from the rock. It’s used in laboratories for acid-base titrations and for producing organic and inorganic compounds like PVC.
We also find substantial use of Hydrochloric Acid across many other industries like:
- Mining,
- water treatment,
- oil/gas,
- detergents,
- leather, building,
- textile,
- rubber,
- photography.
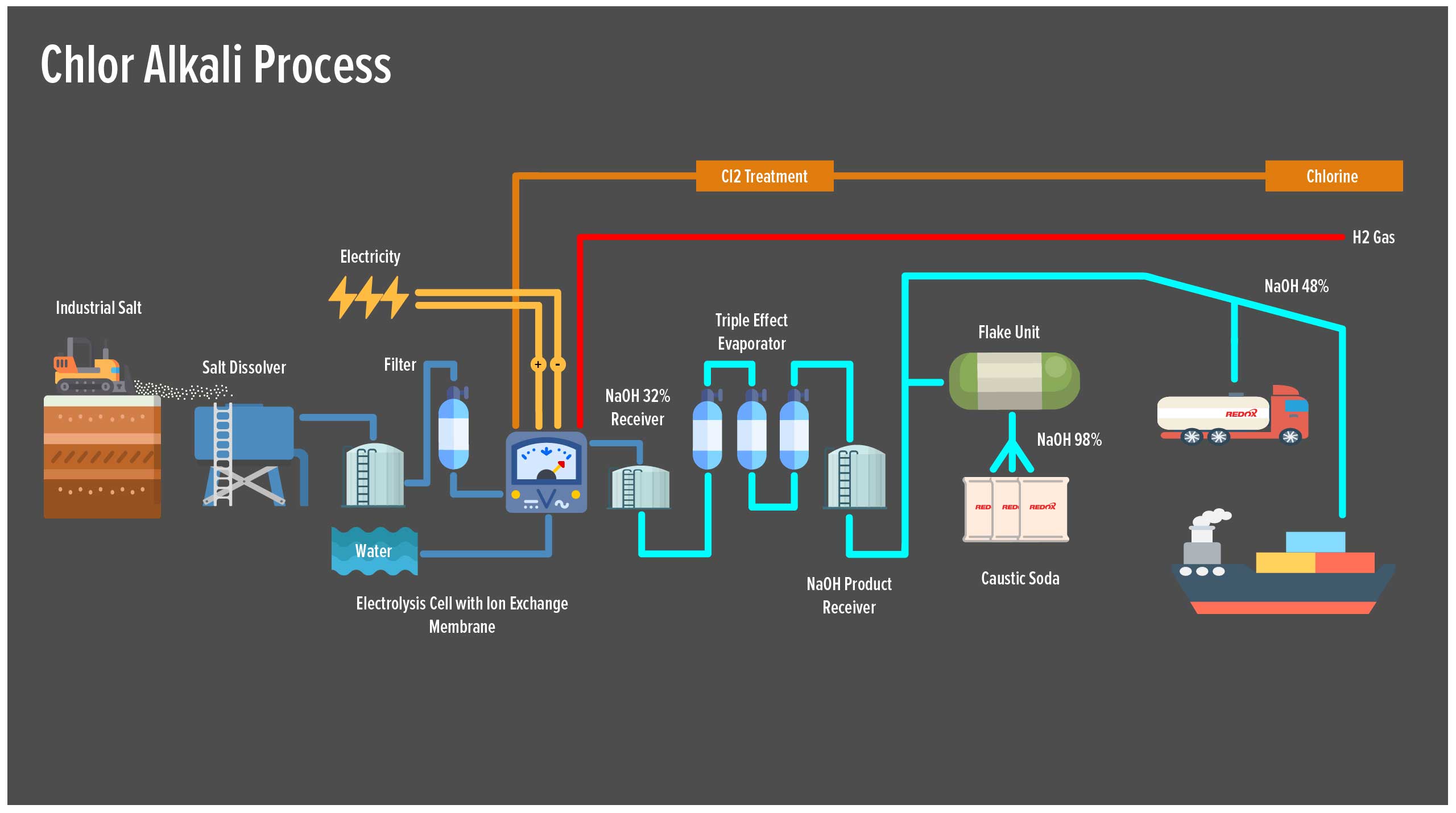
Hydrochloric acid is manufactured predominantly in industrial chlor-alkali plants around the world. The process involves the electrolysis of sodium chloride (salt) solution . This produces chlorine gas , sodium hydroxide and hydrogen gas. The hydrogen is then used to produce hydrochloric acid and ammonia.
In 2020 the global Hydrochloric Acid market size was US $7.8 billion and was expected to record a revenue CAGR of 1.5% over the forecast period through 2028.
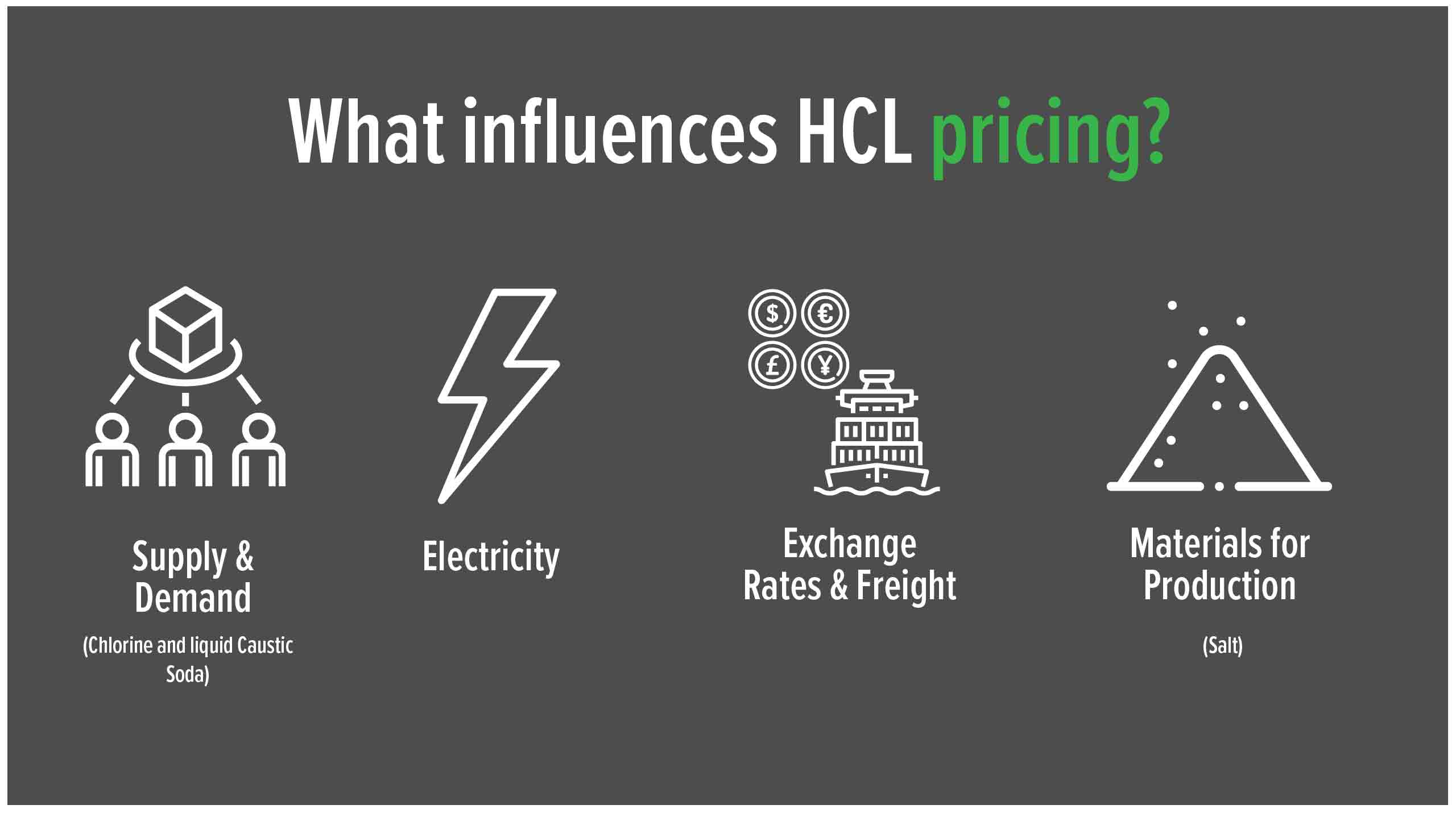
HCL prices are usually fairly stable and tend to increase yearly as a result of CPI increases, usually as a result of the cost of production (labour /electricity costs etc). However, the imported cost of the product is highly dependent on exchange rate variations, packaging and sea freight costs. Finally the overall economics of supply and demand would also play a role in the change in the price of HCL, which is also dependent on Chlorine and liquid Caustic Soda demand, which are all part of the chlo-alkali process.
Hydrochloric Acid In The Home
In the home, you’re most likely to find it used in cleaning agents like toilet and tile cleaners, as it removes grime without reacting to many bathroom surfaces.
Is Hydrochloric Acid Corrosive?
In short, yes. Hydrochloric Acid is corrosive to organic tissues and will corrode mucous membranes, eyes, skin. It can also be corrosive to almost all metals.
How can we help you?
Redox’s Hydrochloric Acid is available in various pack sizes, including 20-litre carboys, 240 kg drums, 1150 kg IBCs and bulk tanker/Isotainers loads. The product comes in a range of strengths ranging from 6% to 33%, with 32% hydrochloric acid being the main commonly used strength.
Contact one of our experts to discover how Redox can be essential to your sourcing strategy.
The Water Industry Operators Association of Australia (WIOA) is the peak national body for those working in operational roles in the water sector. .
With its increasing national membership base, WIOA assists its members and water industry stakeholders in collecting, generating, and exchanging high-quality operational information.
Each year, WIOA host a variety of operationally focused conferences and exhibitions whose fundamental goals are:
- The distribution of the latest “operational” technical and research-based information through platform and poster presentations.
- Update of knowledge and skills plus network development by operations staff through interaction with fellow Water Industry employees.
- Provide opportunities to view and discuss the latest advances in technical equipment and systems with suppliers and trade consultants.
Redox attends all major WIOA conferences annually and will be there at the Tamworth Regional Entertainment and Conference Centre (TRECC) this April 6th and 7th.
The conferences cover a wide range of industry personnel from all levels of the industry. This includes key decision-makers, engineers, and of course, the operators themselves.
Our team will be at stand 64, and we look forward to seeing you there. Please come by and speak with one of our specialists about how Redox can become an integral part of your sourcing strategy.
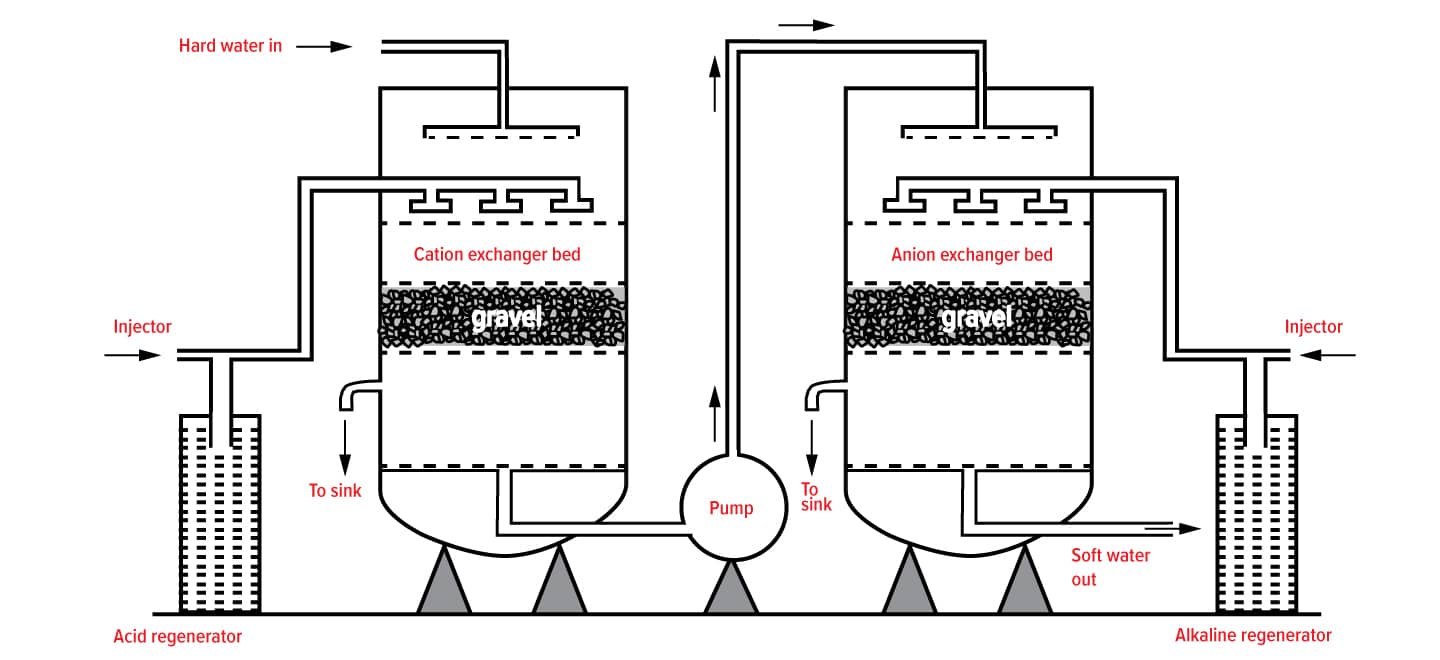
Ion Exchange is a reversible chemical reaction where dissolved ions are removed and replaced with other ions of the same/similar electrical charge. This mechanism is exploited by Ion Exchange Resins which are a medium that facilitates the exchange of ions between the resin beads and the water as it moves past inside a vessel.
This process can create ultra-pure water for use in the production of semiconductors, solar photovoltaics, pharmaceuticals, and flat panel displays for rinsing/cleaning and various other critical processes.
Food manufacturers can use Ion Exchange Resins in a host of ways, from purifying water to use as an ingredient to decolourising, demineralising, and removing taste and odours.
In power stations Ion Exchange Resins are used to demineralise feed water and treat condensate from the steam cycle. They are even utilised to recover various metals such as gold, uranium, and copper.
Possibly the widest use is as a pre-treatment step in various water treatment settings where they can work to improve the efficiency of Reverse Osmosis plants by lessening the load on filter membranes.
The two most commonly used Ion Exchange Resins are:
- Cation resins, that exchange positively charged ions, such as sodium, for calcium,
- Anion resins, which exchange negatively charged ions, such as chloride, for arsenic.
They are used to remove poisonous and hazardous metal ions from solution, replacing them with more innocuous ions, such as sodium and potassium.
How Ion Exchange Resins work
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions.
Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings.
Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium carbonates, bicarbonates and sulphates. Iron oxides or iron carbonates can give a reddish-brown colouration to hard water deposits.
And while The World Health Organization says that “there does not appear to be any convincing evidence that water hardness causes adverse health effects in humans”, there are studies that correlate domestic hard water usage with increased eczema in children.
They can help improve both access and quality by helping provide a higher level of filtration for drinking water in developing countries around the world.
Redox can supply it in 25L and 1000L bags.
Contact us today and speak to one of our industry specialists to find out more.
Our Partnering Manufacturers