Nitrate of soda, scientifically known as sodium nitrate (NaNO3), is a versatile compound that has played a crucial role in various industries throughout history. From agriculture to manufacturing, this compound has proven itself indispensable. Let’s delve into the fascinating story of Nitrate of soda, exploring its uses, historical significance, and impact on different sectors.
Historical Roots
The history of Nitrate of soda is intertwined with the development of the mining industry. Deposits of sodium nitrate were discovered in Chile’s vast and arid landscapes in the early 19th century. The substance was initially known as “Chile saltpeter,” reflecting its origin and economic importance.
As demand grew, Nitrate of soda became a sought-after commodity, sparking a thriving industry in Chile and beyond.

Workers loading nitrate onto ships, Pisagua, Chile, 19th Century. In 1810 large nitrate (salitre or saltpeter) deposits were discovered in the Corregimiento de Tarapaca, and Pisagua became an important port due to its major role in the export of this product.
Agricultural Marvel
One of the primary and enduring uses of Nitrate of soda is in agriculture.
Its high solubility in water makes it an excellent source of nitrogen, a vital nutrient for plant growth. Farmers worldwide have utilised Nitrate of soda as a nitrogen-rich fertiliser to enhance soil fertility and promote robust plant development.
Its effectiveness in providing immediate nourishment to crops has made it a staple in modern agricultural practices.
Industrial and Manufacturing Uses for Nitrate of soda
Nitrate of soda’s applications extend beyond agriculture and explosives. It serves as a reducing agent, decolourising agent, and even a component in producing certain chemicals in various industrial processes.
Its solubility and chemical properties are valuable in diverse manufacturing sectors, including glass, dyes, and metal treatment.
Explosive Applications
During the early 20th century, Nitrate of soda found another critical application – in producing explosives. Ammonium nitrate, a derivative of Nitrate of soda, became crucial in manufacturing explosives and munitions.
This application played a significant role during wartime, highlighting the compound’s versatility in peaceful and wartime industries.
Other industry uses for Nitrate of soda
Some other sectors where sodium nitrate is commonly used include:
- Food Preservation – Sodium nitrate is used as a preservative in cured meats, such as bacon and hot dogs, to prevent the growth of bacteria and extend shelf life. It also helps maintain the colour and flavour of the meat.
- Chemical Industry – Sodium nitrate is used in the chemical industry to produce various chemicals, including nitric acid, a precursor to many chemical compounds.
- Metallurgy – Sodium nitrate is used in specific metal treatment processes, such as surface hardening and corrosion prevention.
- Pharmaceuticals – In the pharmaceutical industry, sodium nitrate can be used to formulate certain medications and pharmaceutical products.
- Textile Industry – Sodium nitrate is used in dyeing and printing textiles, acting as an oxidising agent in some dyeing processes.
- Heat Transfer Fluids – Sodium nitrate is used in some heat transfer fluids, especially in solar thermal power plants, where it serves as a heat storage medium.
- Pyrotechnics Sodium nitrate is used to formulate certain fireworks and pyrotechnic devices as oxidising agents.

By inhibiting bacterial proliferation, sodium nitrate helps extend the shelf life of these cured meat products, contributing to their longevity and maintaining their quality over time.
As you can see, Nitrate of soda stands as a testament to the dynamic relationship between human innovation and the resources our planet provides. From its humble origins in Chile to its widespread applications across industries, Nitrate of soda continues to shape our world.
How can we help?
With extensive, well-established networks in the agricultural, mining, explosives, and other Nitrate-dependent industries, contact us today to delve into competitive pricing for Nitrate of Soda.
Ensure you maintain a leading position in your market and uncover how we can be a crucial partner in shaping your procurement strategy.
One of the most powerful solutions to ensure a safe mine environment is fire retardant anti-static (FRAS) materials, which protects against all kinds of hazardous situations like fuel fires or explosions caused by heat build-up, static sparks, and other combustible substances that could seriously compromise your mine’s safety.
Materials such as rubber, polyurethane, PVC, polyethylene, polypropylene and polyester are often used to make base materials that are then manufactured into products for underground coal mining. Fibre-reinforced resin materials such as fibreglass and carbon fibre composite are also used.
The products manufactured include ventilation sheeting (brattice) and ventilation stoppings, ventilation ducting (rigid and flexible), dust curtains, venturi blowers, air fans, pipes, conveyor belting and conveyor accessories.
Recommendations on FRAS materials
Mine operators of underground coal mines must ensure that control measures are implemented for products subject to the accumulation of static charge. The control measures should include the following:
- Procedures to verify the products are installed per the manufacturer’s directions for static discharge.
- Appropriate measures are implemented to ensure that the properties of FRAS materials are maintained over time.
Manufacturers of FRAS-rated materials must ensure that testing is undertaken in accordance with the relevant requirements of MDG 3608 by an independent testing facility for:
- All FRAS material intended for use in an underground coal mine
- Any change of material composition or manufacturing technique for a FRAS-rated material, including changes of colour, to verify the suitability of the material.
Manufacturers and suppliers of products incorporating FRAS materials as components must ensure that:
- The product’s design incorporates suitable controls to prevent the accumulation of a static charge on the FRAS components.
- Instructions for the safe use of the product include mounting or suspension requirements to prevent the accumulation of a static charge on the FRAS components.
- Any change of material composition or change to the product design, including changes of colour, size or placement of eyelets, rivets or mountings, are tested in accordance with the relevant requirements of MDG 3608
- The product is suitably marked to indicate compliance with MDG 3608.
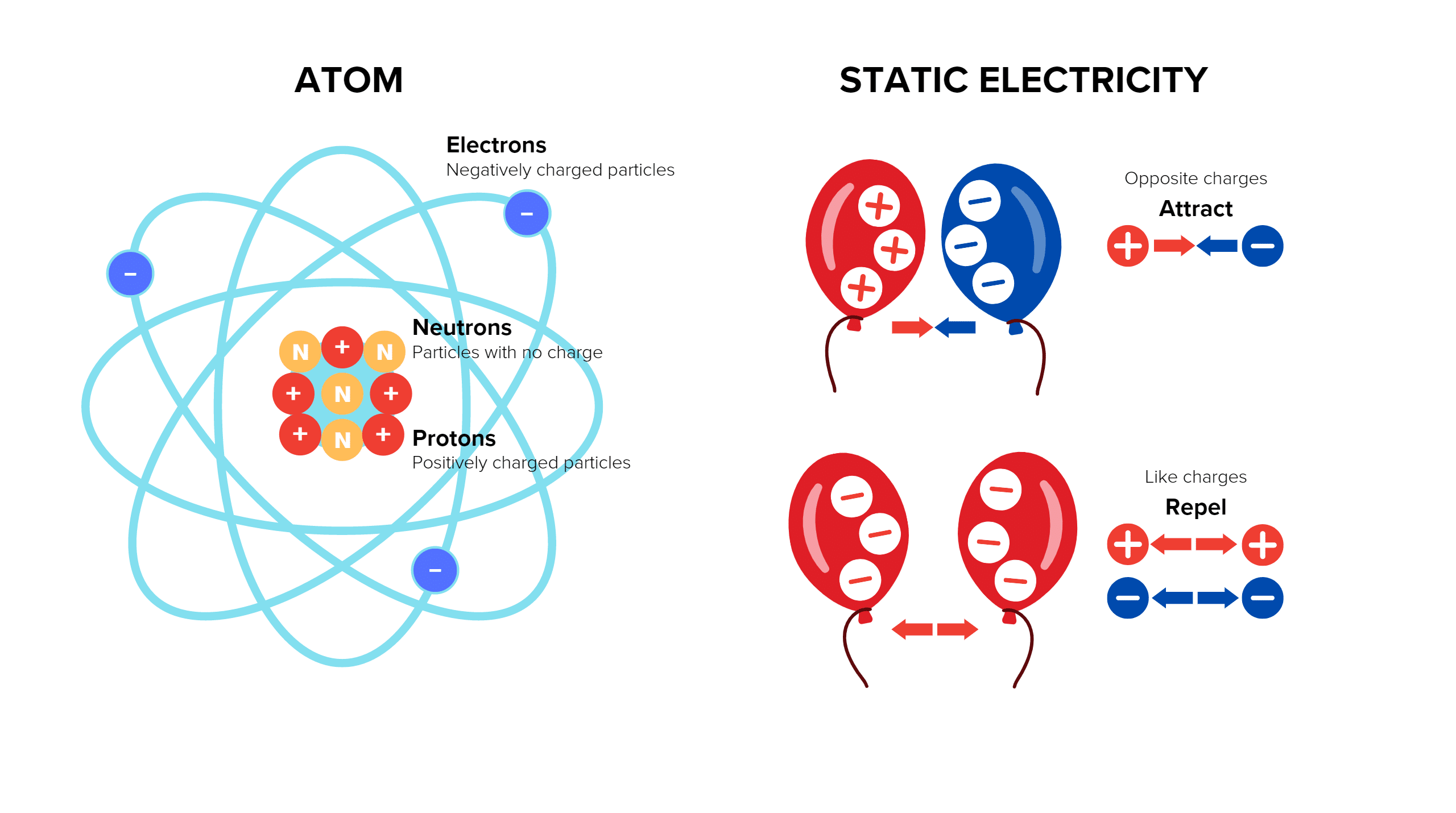
Every object is made of atoms that are typically electrically neutral. They contain an equal number of protons (positive charge) within their nucleus and electrons (negative charge) surrounding the nucleus. Static electricity occurs when there is a separation of positive and negative charges within or on the surface of a material or between materials. Electrons may move from one object to another, leaving one object with a negative charge and the other with a positive. When the objects are separated they retain the charge imbalance.
How can we help?
To minimise risks, improve occupational health and safety hazards, and, importantly, minimise underground ignitions, fire retardant anti-static (FRAS) materials are now required in all NSW underground mines, monitored by the NSW resources regulator.
Redox offers many polymer/composite options for differing FRAS application solutions.
Contact our Plastics division today if you would like to learn more about material solutions supported by our many international principal partners.
Did you know that Hydrochloric Acid is also known as HCL, muriatic acid, or spirits of salt? It’s utilised in a variety of industrial and commercial settings. For those who work in industries that use this chemical, it’s vital to understand the most common applications, what they accomplish, and what you need to know to handle them safely and responsibly.
What Is Hydrochloric Acid?
Hydrochloric Acid is an odourless, colourless solution of hydrogen chloride in water with a pungent smell. But behind this almost invisible veneer lies a powerful punch. For instance, Hydrochloric Acid can react with metals to form an explosive gas. Yet, it can also be found in many home cleaning products.

1150 kg IBCs housed in our warehouse, classified Hazard Class 8, are for corrosive materials, defined as substances that can cause significant harm to living tissue and/or corrode steel and aluminium if they leak.
Hydrochloric Acid is classified as a class 8 hazardous product, i.e. it’s a corrosive substance and can cause burns and irritation to the skin. Due to its corrosive properties, extreme care must be taken when handling this product. Make sure to wear appropriate safety equipment when handling hydrochloric acid. Ensure to avoid direct eye contact; if this occurs, seek immediate medical advice.
We recommend you consult the safety data sheet when using, storing or handling the product.
Hydrochloric Acid in the market and its many uses
This potent acid is found in many industries and has a wide range of uses.
What is Hydrochloric Acid used for?
The most significant end uses for Hydrochloric Acid are cleaning, the productions of fertilisers and dyes, steel pickling, oil well acidising, food manufacturing, producing calcium chloride, and ore processing.
How it’s used also varies significantly. For instance, in water treatment, it’s used to control pH levels, or in swimming pools, it can help remove any stubborn algae from the floors and walls of your pool. In acidising oil wells, it helps remove carbonate reservoirs, or limestones and dolomites, from the rock. It’s used in laboratories for acid-base titrations and for producing organic and inorganic compounds like PVC.
We also find substantial use of Hydrochloric Acid across many other industries like:
- Mining,
- water treatment,
- oil/gas,
- detergents,
- leather, building,
- textile,
- rubber,
- photography.
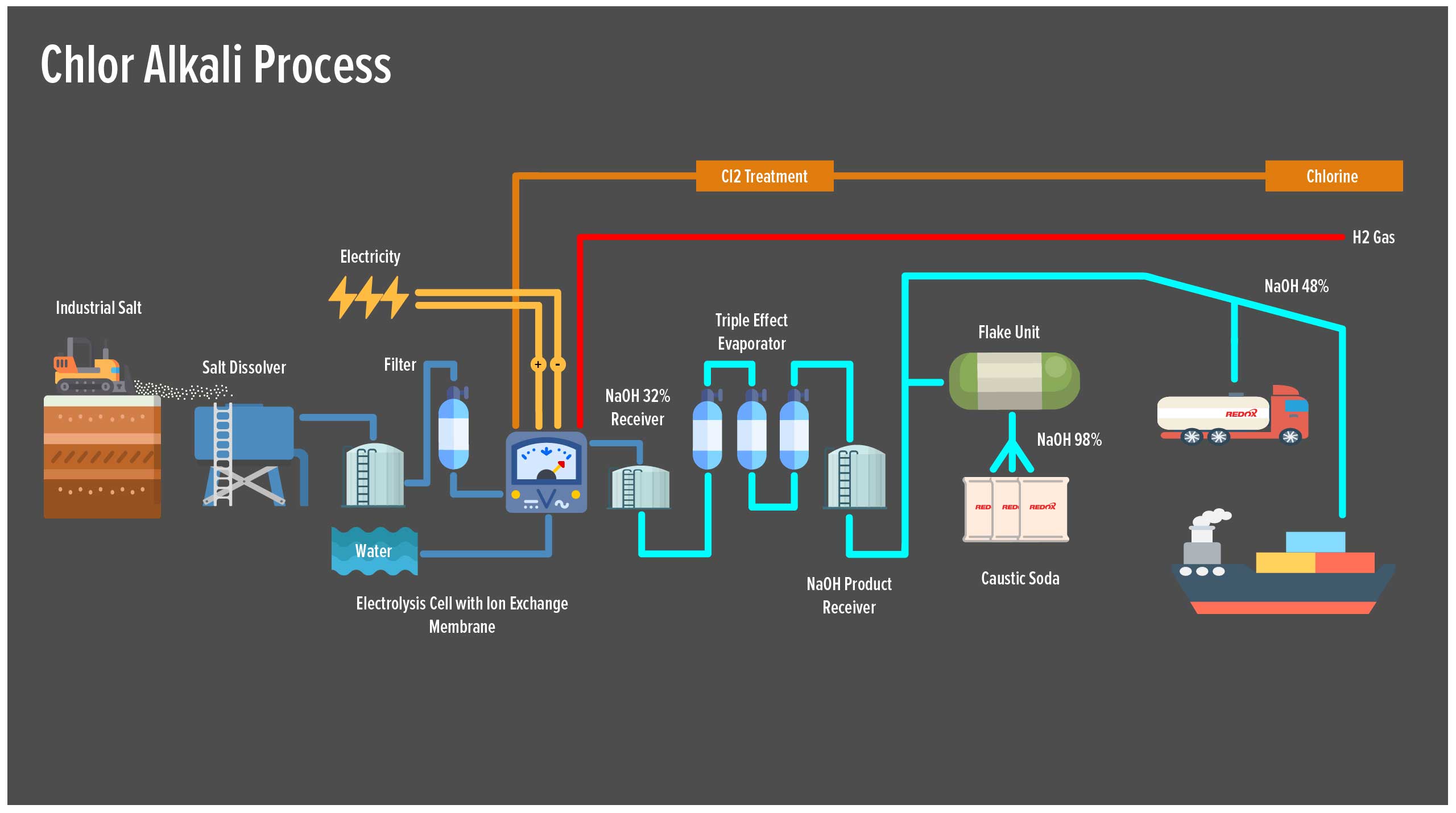
Hydrochloric acid is manufactured predominantly in industrial chlor-alkali plants around the world. The process involves the electrolysis of sodium chloride (salt) solution . This produces chlorine gas , sodium hydroxide and hydrogen gas. The hydrogen is then used to produce hydrochloric acid and ammonia.
In 2020 the global Hydrochloric Acid market size was US $7.8 billion and was expected to record a revenue CAGR of 1.5% over the forecast period through 2028.
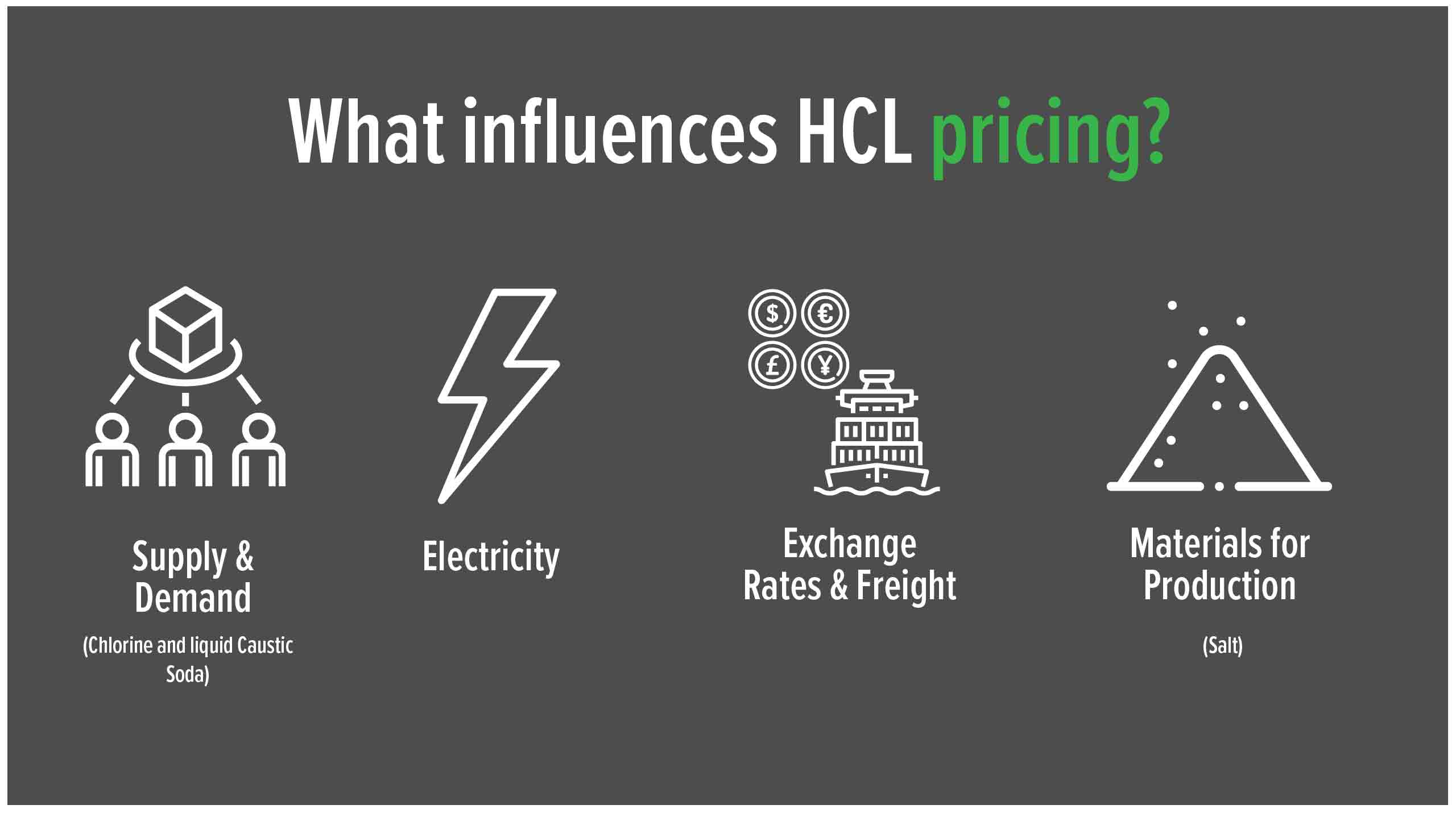
HCL prices are usually fairly stable and tend to increase yearly as a result of CPI increases, usually as a result of the cost of production (labour /electricity costs etc). However, the imported cost of the product is highly dependent on exchange rate variations, packaging and sea freight costs. Finally the overall economics of supply and demand would also play a role in the change in the price of HCL, which is also dependent on Chlorine and liquid Caustic Soda demand, which are all part of the chlo-alkali process.
Hydrochloric Acid In The Home
In the home, you’re most likely to find it used in cleaning agents like toilet and tile cleaners, as it removes grime without reacting to many bathroom surfaces.
Is Hydrochloric Acid Corrosive?
In short, yes. Hydrochloric Acid is corrosive to organic tissues and will corrode mucous membranes, eyes, skin. It can also be corrosive to almost all metals.
How can we help you?
Redox’s Hydrochloric Acid is available in various pack sizes, including 20-litre carboys, 240 kg drums, 1150 kg IBCs and bulk tanker/Isotainers loads. The product comes in a range of strengths ranging from 6% to 33%, with 32% hydrochloric acid being the main commonly used strength.
Contact one of our experts to discover how Redox can be essential to your sourcing strategy.
Flotation reagents are a crucial processing technology that has remained relatively unchanged since its introduction, though It has seen numerous advancements in both use, technology, and reagents over the years.
Then and now
Initially employed in the mining and mineral processing sectors, it was one of the most significant 20th century enabling technologies. The ancient Greek, Persian, and Egyptian writings are among the earliest indications of this technology’s antiquity.
However, contemporary flotation methods are more a testament to significant technological advancements in flotation chemistry and chemicals than hardware and manual labour.
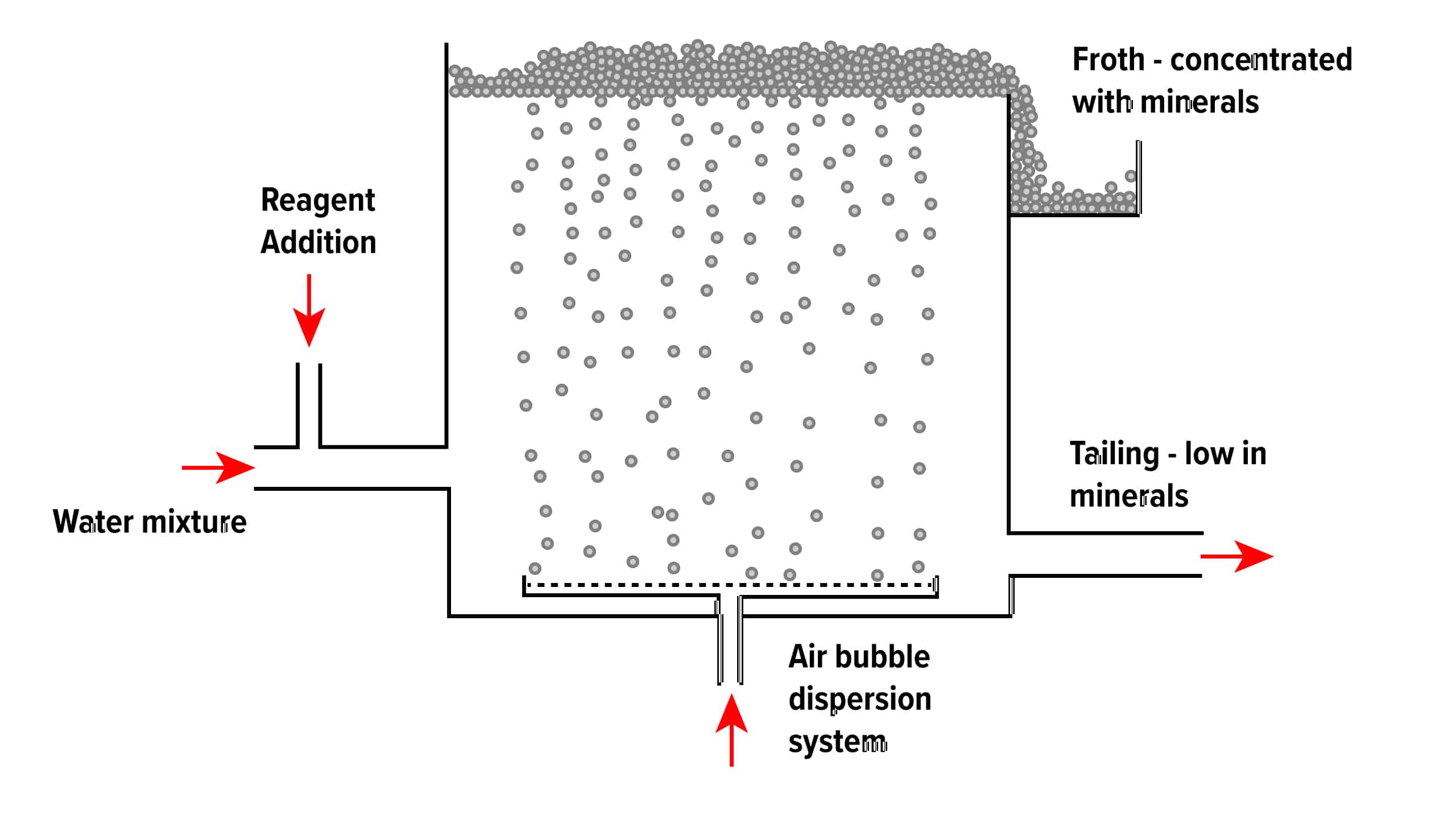
The froth flotation process is about taking advantage of the natural hydrophobicity of liberated (well ground) minerals/metals and making/playing on making them hydrophobic (water-repel) individually to carefully separate them from one another and the slurry they are in.
Mineral Processing
Froth flotation is a method of separating minerals from gangue by exploiting differences in hydrophobicity. Wetting agents and surfactants are used to make the hydrophobic distinctions between valuable material and waste gangue possible.
The selective separation of the minerals makes processing mixed ores a more economically feasible task. It is particularly effective in treating fine minerals and solves the complex recovery process of valuable components in many fine mineral particles. The flotation process separates an extensive range of sulphides, carbonates and oxides before further refinement.
Flotation has advanced considerably in the last decade, introducing froth flotation equipment, froth flotation reagents, and froth flotation technology. These advances have contributed to more than 60% – 70% of the ore in the world being separated by the froth flotation method.
The development of froth flotation has improved the recovery of valuable minerals, such as copper- and lead-bearing minerals. Along with mechanized mining, it has allowed the economic recovery of valuable metals from much lower grade ore than previously possible.
Some of the advantages of the froth flotation method include:
- It’s one of the most popular methods, which enjoys a near-monopoly in several mineral processing technologies.
- It can be used to process a variety of nonferrous metals, rare metals, and non-metallic minerals.
- High separation efficiency, especially suitable for low grade, fine grain size minerals.
Different Classifications
Flotation reagents are classified according to the role they play in flotation. Collectors, frothers, regulators, and depressants are used as classifications for such reagents.
- The collector’s function is to take from a hydrophobic layer on a mineral surface in the flotation pulp and create conditions for the attachment of the hydrophobic particles to air bubbles and recovery of particles in the froth product.
- Surface-active frothers are heteropolar surface-active chemicals that decrease the surface tension of water and have the ability to adsorb on the air bubble–water interface. Their presence in solution increases the film strength of air bubbles, allowing for better hydrophobic particle attachment.
- Activators, depressants, and pH regulators are often referred to as modifiers or regulators of the flotation process. The main purpose of these reagents is to modify the action of the collector on mineral surfaces and therefore govern the selectivity of the flotation process. In the presence of regulators, the collector only adsorbs on particles that are targeted for recovery.
How can we help?
It’s essential to work with a supplier that can guarantee consistent quality; Redox can supply within Australia, New Zealand, and the United States and is in a great position to meet and exceed your expectations; contact one of our industry specialists today.
Gold refining is a process that has been around for centuries and is necessary in producing a high purity product having both value as jewellery and for investment. Prior to purchasing gold in a store, there are many steps that take place before that special investment becomes available to you.
A mining operation requires extensive exploration and development before they can offer gold for refining. On average, it takes 10-20 years of work before an operation is ready for production.
Processing ore to extract the precious metal is the first step in this chain of events, which is followed by recovery and refining in a sequence of processes to achieve the purity required.
Redox Reagent Products
Redox reagent products have been successfully used by our customers for many years. Recently one of our newest clients produced their first bars of gold Doré using our many high-quality reagents.

The reagents worked well as the bar had a very nice clean separation between the yellow gold and the dark grey/black slags. This gold is nice and yellow, a sign of few impurities.
Redox regents were efficient in removing copper and silver that enabled our client to refine 95-99% pure gold from the starting material.
The reagents used were of such high quality that they enabled a great quality flux, in much the same way as making a great cake starts with quality ingredients – so should gold be refined from high-quality fresh ingredients to produce a better product.
Some of the reagents used were:
- Silica Flour
- Borax Pentahydrate
- Soda Ash
Our reagents were used to refine the metal bar product and it appears from the above image that the operators were skilled, as seen in the clean separation of yellow gold against dark grey slags. This indicates a nice quality of ore, likely containing less copper than other ores, which would result in a rose gold-like appearance.
Redox offers client’s access to chemical additives which cover the entire vertical integration process of gold production such as:
Typical reagents for gold rooms
- Silica flour
- Anhydrous Borax
- Soda Ash
- Potassium Nitrate
Cyanide destruction reagents
- Ferrous sulphate
- Hydrogen Peroxide
- Sodium metabisulphite
Other reagents
- Activated Carbon
- Sodium Cyanide
- Lead nitrate
- Anti-scalants
- Flocculants
- Coagulants
- Collectors
- Frothers
- Caustic soda solution
- Hydrochloric acid
- Nitric Acid
- Sulphuric acid
Have you considered Glycine?
Redox is a large and experienced supplier of glycine for the Australian market. Glycine is an emergent technology which in some circumstances eliminate the need for cyanide destruction as it prevents the formation of WAD (weak acid dissociable) cyanide whilst simultaneously improving leaching kinetics in difficult to process ores such as reactive iron sulphides or copper rich ores.
Want to know more? Contact one of our industry specialists today and ask them about our wide range of regents.
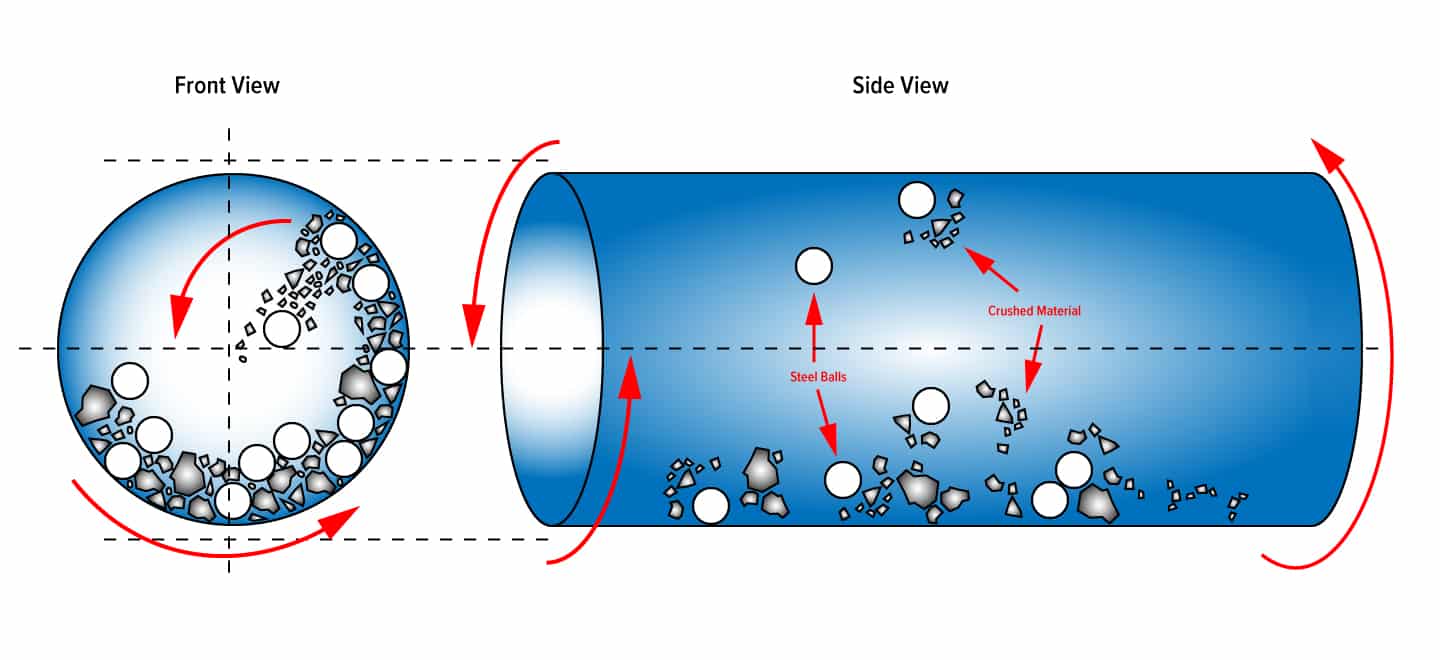
The general idea behind the ball mill and grinding media is an ancient one, but it was not until the industrial revolution and the invention of steam power that an effective ball milling machine could be built.
Grinding Media is primarily used in ball milling (ore processing) and regrind (cement or similar) applications. The critical factor to quality and reliability is the hardness or “wear rate” of the ball media being used. Where there are other forms like rods and cyplebs, the ball is the most common.
The three major types of ball media are:
- Forged Steel
- Chrome
- Ceramic
For the ore processing application, most mines will have at least 2 ball mills; a primary and a secondary and occasionally some will utilise a regrind mill.
In the primary and secondary, either forged steel or chrome is used; rarely do they use a combo of both.
In the regrind mill; generally ceramic media is used.
Sizes vary depending on the intended application:
- Forged steel and chrome media come in sizes from 25mm to 140mm most commonly.
- Ceramic will come in 1-22mm; most commonly 1-5mm.
Chrome is broken into “low content chrome” and “high content chrome”. Low content chrome historically has smaller ball size and can replace the need for regrind mill altogether.
Want to know more? Contact one of our industry specialists today and ask them today.
Our Partnering Manufacturers