Ion Exchange is a reversible chemical reaction where dissolved ions are removed and replaced with other ions of the same/similar electrical charge. This mechanism is exploited by Ion Exchange Resins which are a medium that facilitates the exchange of ions between the resin beads and the water as it moves past inside a vessel.
This process can create ultra-pure water for use in the production of semiconductors, solar photovoltaics, pharmaceuticals, and flat panel displays for rinsing/cleaning and various other critical processes.
Food manufacturers can use Ion Exchange Resins in a host of ways, from purifying water to use as an ingredient to decolourising, demineralising, and removing taste and odours.
In power stations Ion Exchange Resins are used to demineralise feed water and treat condensate from the steam cycle. They are even utilised to recover various metals such as gold, uranium, and copper.
Possibly the widest use is as a pre-treatment step in various water treatment settings where they can work to improve the efficiency of Reverse Osmosis plants by lessening the load on filter membranes.
The two most commonly used Ion Exchange Resins are:
- Cation resins, that exchange positively charged ions, such as sodium, for calcium,
- Anion resins, which exchange negatively charged ions, such as chloride, for arsenic.
They are used to remove poisonous and hazardous metal ions from solution, replacing them with more innocuous ions, such as sodium and potassium.
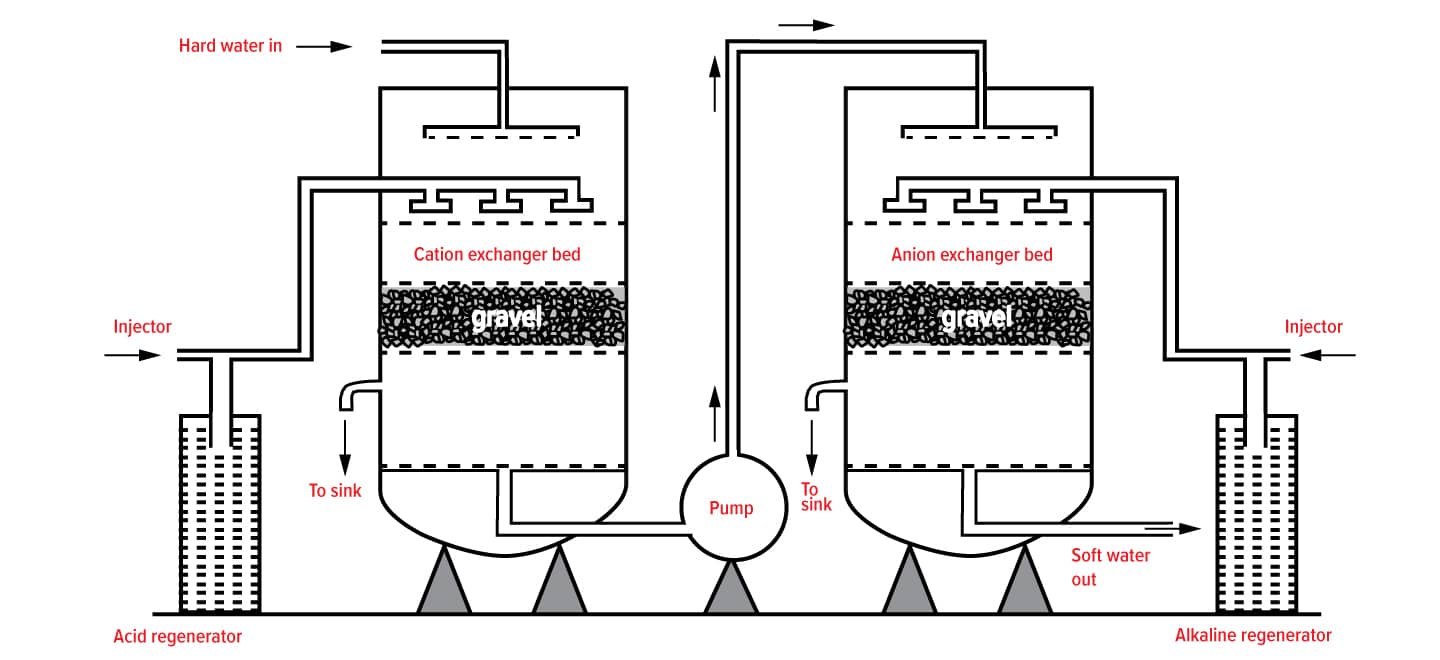
How Ion Exchange Resins work
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions.
Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings.
Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium carbonates, bicarbonates and sulphates. Iron oxides or iron carbonates can give a reddish-brown colouration to hard water deposits.
And while The World Health Organization says that “there does not appear to be any convincing evidence that water hardness causes adverse health effects in humans”, there are studies that correlate domestic hard water usage with increased eczema in children.
They can help improve both access and quality by helping provide a higher level of filtration for drinking water in developing countries around the world.
Redox can supply it in 25L and 1000L bags.
Contact us today and speak to one of our industry specialists to find out more.