A surfactant, also known as a surface-active agent, is a substance that lowers the surface tension between two substances. It is a molecule that contains both hydrophilic (water-loving) and hydrophobic (water-hating) parts, allowing it to interact with water and oil.
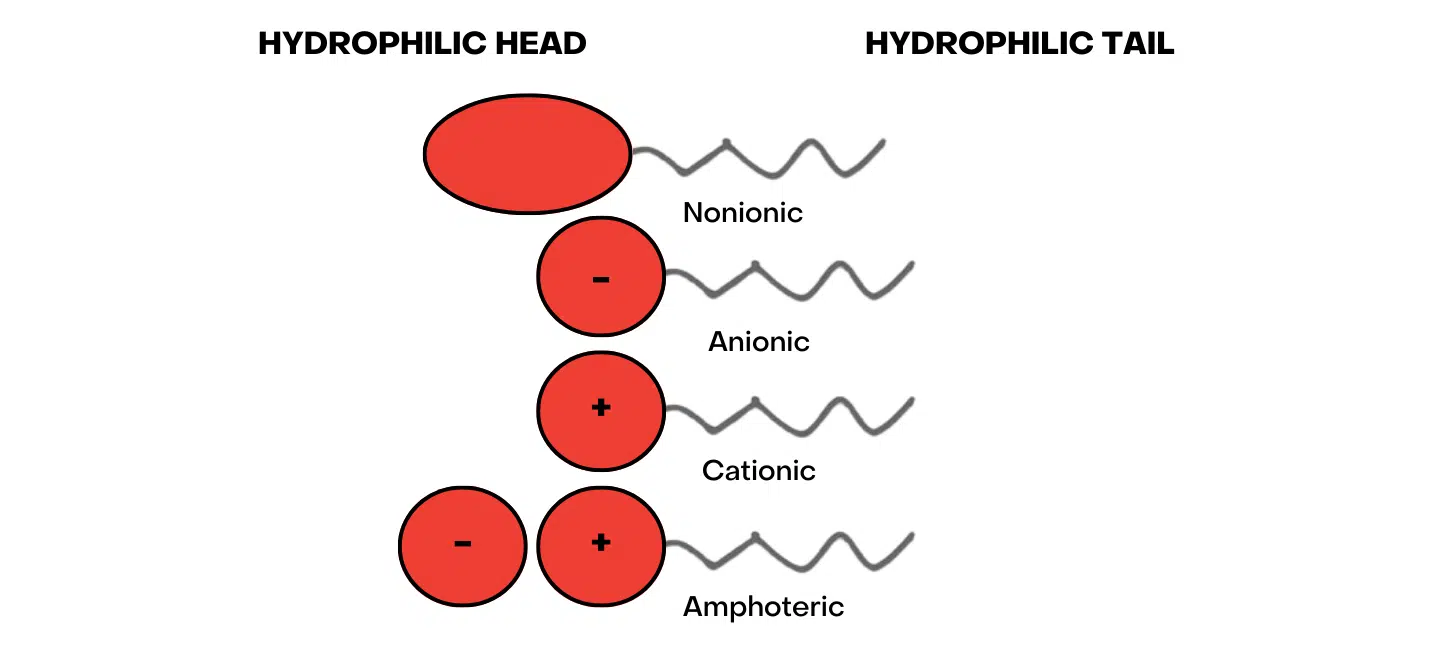
Surfactants are a wide spectrum of products but are broadly classified as Anionic, Nonionic, Cationic or Amphoteric depending on whether the electrical charge is positive, negative or neutral on the hydrophilic head.
Surfactants are commonly used in cleaning products such as detergents, soaps, and shampoos. They can break down oils and dirt and help them mix with water for easier removal. They are also used in industrial processes such as oil recovery and in medical applications such as lung surfactants, which help maintain the structure of the lungs and prevent collapse.
Examples of surfactants include sodium lauryl sulphate, commonly found in household cleaning products and personal care items, and polysorbate 80, used in food, cosmetics, and pharmaceuticals.
Surfactants are used in a wide variety of products, including:
- Detergents and cleaning products: Laundry detergents, dishwashing liquids, all-purpose cleaners, and bathroom cleaners all contain surfactants that help to remove dirt, oil, and grease from surfaces.
- Personal care products: Shampoos, body washes, hand soaps, and toothpaste all contain surfactants that help to clean and remove oils from the skin and hair.
- Cosmetics: Makeup removers, facial cleansers, and body wash contain surfactants that help break down and remove makeup and other impurities.
- Food and beverages: Emulsifiers and stabilizers are surfactants used in food and beverage products to help mix ingredients that would not usually mix well, such as oil and water.
- Agriculture: Surfactants are used in agricultural products, such as herbicides and pesticides, to help them stick to plants and improve their effectiveness.
- Petroleum: Surfactants are used in the petroleum industry to improve oil and gas flow through pipelines and help recover oil from underground reservoirs.
- Lubricants: By adding surfactants to car engine lubricants, it becomes possible to prevent particles from adhering to engine parts, enabling the parts to move smoothly and maintain the proper functioning of the car.
Surfactants have several unique qualities that make them useful in a wide range of applications:
- Surface tension reduction: Surfactants have the ability to reduce the surface tension between two substances, such as water and oil. This allows the substances to mix more easily and enhances the ability of the surfactant to clean, emulsify, or disperse.
- Amphiphilic nature: This unique structure allows surfactants to interact with water and oil hydrophilic (water-loving) and hydrophobic (water-hating), essential for their cleaning use and emulsifying.
- Self-assembly: Surfactants can self-assemble into organized structures, such as micelles or bilayers, in certain conditions. This allows them to form stable emulsions, which are essential in creating many products such as lotions, creams, and paints.
- Interfacial activity: Surfactants are active at interfaces, such as the boundary between two liquids or a liquid and a solid. They can adsorb to the surface of a substance, which can change its surface properties, such as its wetting or adhesion behaviour.
Overall, these unique properties make surfactants extraordinarily versatile and valuable in a wide range of applications, from cleaning products and personal care items to industrial processes and medical applications.